
Computer System Validation (CSV)
Computer System Validation (CSV) ensures that computer-based systems consistently perform as intended while meeting regulatory standards such as FDA 21 CFR Part 11, GxP, and EU Annex 11 throughout their lifecycle.
What is Computer System Validation?
CSV is a systematic, documented process that confirms a computer system performs accurately, reliably, and consistently while meeting regulatory requirements. It ensures the integrity of electronic records and electronic signatures.
Why Computer System Validation Matters
CSV ensures that regulated industries such as pharmaceuticals, healthcare, life sciences, and medical devices operate with accuracy, compliance, and data integrity.
Key reasons CSV is important:
- Ensure Compliance – Meets FDA 21 CFR Part 11, EU Annex 11, and global regulatory standards.
- Enhance Data Integrity – Guarantees that data is complete, consistent, and protected.
- Mitigate Risks – Identifies potential failures early to prevent compliance risks and system issues.
What We Offer
Regulatory Compliance
Full compliance with FDA 21 CFR Part 11, EU Annex 11, GAMP5, and GxP standards.
Complete CSV Documentation
Validation Plans, URS, FRS, IQ/OQ/PQ protocols, Traceability Matrix, and summary reports.
Ongoing Revalidation
Continuous system revalidation as your system evolves, ensuring long-term compliance.
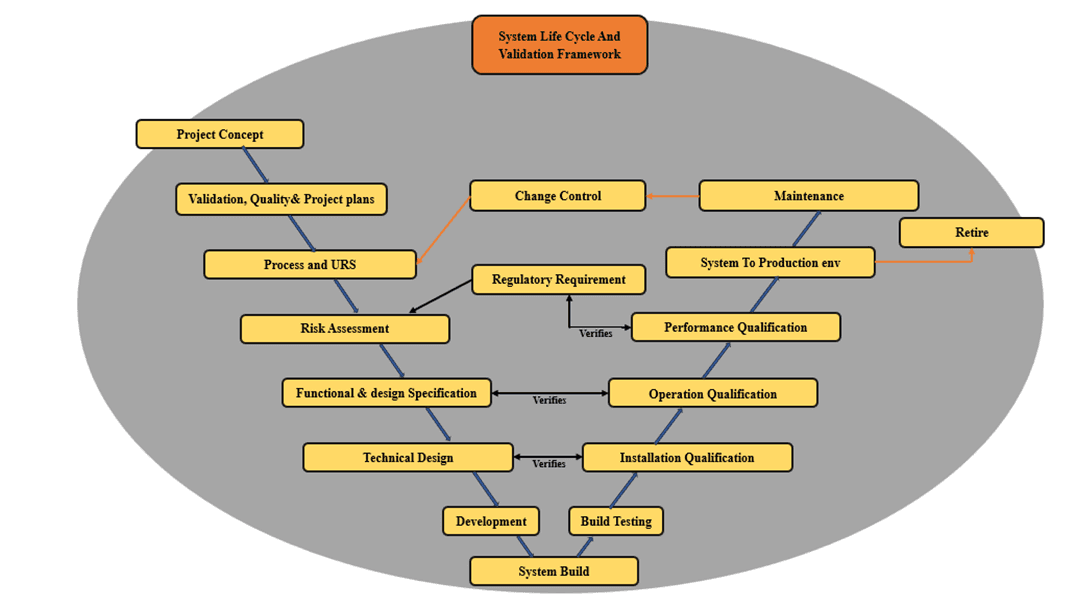
Our Validation Process
Planning & Documentation
We create validation plans, scope, user requirements, and documentation strategies.
Requirements Gathering
We collaborate with your team to define User Requirements (URS) and system expectations.
Risk Assessment
We analyze risks to prioritize validation activities and minimize compliance gaps.
Validation Testing (IQ/OQ/PQ)
We perform Installation Qualification, Operational Qualification, and Performance Qualification.
Documentation & Reporting
Every step is thoroughly documented to maintain audit-ready compliance.
Training & Support
Your team receives training to manage CSV processes and maintain compliance.
Revalidation & Continuous Support
We ensure your system stays compliant as it updates or scales over time.
FAQs
What is included in Computer System Validation?
CSV includes planning, risk assessment, requirements, IQ/OQ/PQ testing, documentation, reporting, and continuous validation support.
Which industries need CSV?
Industries such as pharmaceuticals, medical devices, biotechnology, and healthcare are required to follow CSV guidelines.
Does Techvalio support FDA 21 CFR Part 11 compliance?
Yes. Our CSV process ensures full compliance with 21 CFR Part 11, EU Annex 11, and other GxP standards.
